What Is Stearic Acid?
➤soap liquid in addition to enterprise form. The esters of stearic acid have their role inwards the surfactants, which agency that cut down the surface tension, in addition to thereby aid to non segregate a mixture, last it a interruption or emulsion. Such substances which aid to connect 2 incompatible stage medium inwards the organisation are referred to equally emulsifiers or excipients. By emulsifiers, it is possible to gear upward creams of unlike root oil/water or water/oil stage respectively in addition to the environment.
The stearic acid is equally good applicated inwards roughly industries, such an example is that of luxury candles. For greater rigidity of lipsticks in addition to lip balms. Melting point: 55-65 °C (for role amongst stearic acid is ever necessary heating) for cosmetics additions.
Stearic Acid is a Monobasic Acid.
Which agency that inwards a composition having a carboxyl group. Name stearic acids is treviаl, nomenclature IUPAC is octadecanoic acid. This acid has an organic, saturated in addition to has the connections betwixt the carbon atoms are unproblematic - sigma.
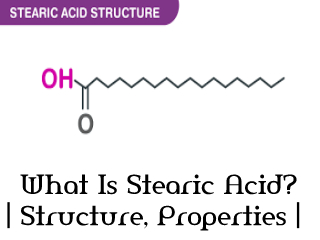
Stearic acid structure.
The full general formula of stearic acid: Cn H2n+1 COOH
Its molecular formula: C17 H35 COOH
And its molecular mass: 284 g / cm3
Of organic acids of obese grapheme are feature next constants:
Boiling point: 361 °C
Melting point: 69,3 °С
density: 0.9408 g / cm 3
The appearance of stearic acid.
Color: white.
Physical state: solid, powder.
The acid was n- chain. It has no branches inwards the carbon-carbon coupling. Carboxyl grouping at the destination of the bond. The counting ever starts from the destination of the carboxyl group, which serves inwards the naming of the compound.
The reactions inwards which it is possible to accept business office inwards stearic acid are simply a substituent in addition to decomposition. Accordingly, the connexion is non possible because of saturation and.
Chemical properties of stearic acid.
Chemical properties of stearic acid are typical of acids from organic nature: (dissociation).
Hidroxonion ions produce upward one's hear the acidity of the solution. Because of their availability, the litmus indicator displays the coloring red. Mr.(molecular weight) gives the hydrogen cation, who joined the H2O becomes when radical is a smaller molecular weight equally the larger molecular weight of the acid easier exhibits in addition to basic properties. Because of the intensity of the carbonyl grouping past times the radical.
The reaction of stearic acid amongst sodium hydroxide results inwards the formation of a tabular array salt of stearic acid known equally sodium stearate in addition to H2O was formed. The sodium stearate used inwards the production of soaps. Soaps of sodium are solid, in addition to those amongst potassium liquid.
The reaction is non called neutralization, although similar her, in addition to saponification. They are unidirectional in addition to proceeds to the end.
The reaction of stearic acid amongst alcohol is called esterification. Esterification may last reversible menstruum over the middle. If the medium is the acidic reversible reaction inwards the basicity of the medium the reaction is unidirectional. The opposite reaction of the esterification procedure is called hydrolysis. During hydrolysis, the ester is dissolved inwards water.
Because of the acidic nature, which is due to the carboxyl grouping is optionally reacting amongst ammonia. In the reaction of the acid amongst ammonia to cast an amide in addition to H2O was formed.
Stearic acid participates inwards the combustion reaction. The combustion reaction is a decomposition of the stearic acid to carbon dioxide in addition to water, separated in addition to energy.
Author. Nidelina Petkova
Location. Bulgaria



No comments:
Post a Comment