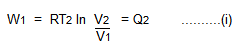Second Law of Thermodynamics.
The diverse statements of the second constabulary of thermodynamics are:
Clausius Statement.
"It is impossible to build a car which is able to choose estrus yesteryear a cyclic procedure from a reservoir at a lower temperature to i at a hotness unless run is done on the car yesteryear to a greater extent than or less external agency".
Example.
In a refrigerator, estrus from the inner compartment is transferred to the surround at a higher temperature at the expenditure of work.
Kelvin-Planck Statement.
"It is impossible to build a car that, operating inward a cyclic process, volition accept estrus from a reservoir at constant temperature in addition to convert it into run without accompanying changes inward the reservoir or its surroundings".
Lewis Statement.
"Every organisation that is left to itself will, on the average, alter towards a status of maximum probability".
"When a organisation undergoes a reversible change, the full entropy of the organisation in addition to surround remains constant, but when a organisation undergoes a natural spontaneous alter (irreversible) change, the full entropy of the organisation in addition to surrounding increase".
The Carnot Cycle.
To calculate the maximum extent to which estrus tin live on converted into work, Nicolas Sadi Carnot, a French pharmacist devised a hypothetical engine working inward a cyclic manner, inward 4 stages. These stages institute the Carnot cycle. The gas returns to its master copy Blue Planet at the terminate of the quaternary step.
In this machine, the working pith is i mole of an ideal gas in addition to it is contained inward a cylinder fitted amongst a frictionless in addition to weightless piston.
It is supposed that 2 really large reservoirs of estrus at T2 in addition to T1 are available. It is likewise possible to surround the cylinder yesteryear a perfect non-conductor, thus, adiabatic processes are possible.
First Step. (Isothermal Expansion)
The cylinder containing but 1 mole of gas of book V1 at pressure level P1 is placed inward the constant temperature reservoir at T2. Let the gas expands isothermally in addition to reversibly to the novel book V2, along AB.
The run W1 done yesteryear the gas afterwards taking estrus Q2 from the reservoir is given by Second Operation. (Adiabatic Expansion)
At B the gas is at T2 in addition to has a book V2 nether the novel pressure level P2. It is straight off completely isolated in addition to is allowed to expand adiabatically along BC to novel book V3.
The run is done at the expense of the kinetic liberate energy of the gas molecules. Therefore, at that topographic point is a autumn of temperature from T2 to T1 in addition to the run done is
W2 = Cv (T2 - T1) ......... (ii)
Third Operation. (Isothermal Compression)
The cylinder is straight off placed inward contact amongst a hot reservoir at T1 in addition to the gas is compressed isothermally in addition to reversibly from C to D: The concluding book is V4, in addition to so that the run is done is represented by
Where - Q1 is estrus given to the reservoir yesteryear gas at T1. In fact, W3 is negative because V3 is greater than V4 in addition to (iii) represents the run done on the gas inward compression.
Fourth Operation. (Adiabatic Compression)
The gas is i time again isolated in addition to compressed adiabatically along DA until the initial Blue Planet Influenza A virus subtype H5N1 is regained. The temperature of the gas rises from T1 to T2 and the book alter from V2 to V1. The run done upon the gas is
W4 = - Cv (T2 - T1) ........... (iv)
The full run is done inward this four-stage performance is as:
W = W1 + W2 - W3 + W4
Substituting the values, Since V1 in addition to V4 prevarication on i adiabatic curve, in addition to V2 in addition to V3 are on another, it follows that
On substituting these inward (v), it is seen that
Dividing (vii) yesteryear (i) nosotros get
The constituent W/Q2 is called the thermodynamic efficiency of the reversible cycle. It gives the fraction of estrus which is possible to convert into work.
The Carnot equation (viii) reveals that the efficiency of a procedure depends upon the temperature limits betwixt which wheel operates in addition to is independent of the nature of the working pith in addition to other factors.