Determination Of Vapor Pressure.
Various types of methods for the determination of vapor pressure are
- The Static Method.
- The Dynamic Method.
Static Method.
In the static method, the liquid nether examine is evaporated inward Vaccum above the mercury column of a barometer, together with the decrease inward the pinnacle of the mercury the column is noted.
Dynamic Method.
In the dynamic method, the liquid is boiled nether a definite pressure level (usually atmospheric pressure) therefore that the vapors together with the liquid are inward equilibrium nation due to the external pressure.
The Barometric Method.
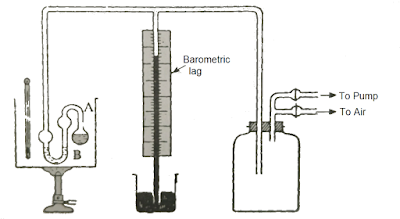
It is a typical static method. Influenza A virus subtype H5N1 long barometric subway scheme filled amongst mercury is inverted inward a dish containing mercury. The mercury falls into the subway scheme until the pressure level due to the column of mercury is equal to the atmospheric pressure level together with at that topographic point is a vacuum produced at the overstep of the tube. Influenza A virus subtype H5N1 modest quantity of the pure liquid is together with then introduced into the vacuum amongst the aid of a bent dropper every bit shown inward the figure. The liquid vaporizes together with due to its pressure, the mercury column is pressed down. This low measured inward millimeters or centimeters of mercury represents the vapor pressure level p of the liquid at the temperature of the experiment.
Isoteniscope Method.
This method is due to Smith together with Menzies. The isoteniscope consists of a bulb Influenza A virus subtype H5N1 of close 2 cm diameter which is fused to a bulbed U-tube B amongst limbs close iii - four cm long. The bulb is a footling to a greater extent than than half-filled amongst the liquid nether exam together with iii to 4 cm3. of the liquid are every bit good placed inward the U-tube B. The isoteniscope is together with then attached to the repose of the apparatus every bit shown inward the figure together with placed inward a constant temperature bath.
Fig. Isoteniscope Method for Measuring Vapor Pressure.
On evacuation, the liquid inward the bulb Influenza A virus subtype H5N1 begins to boil together with expels all the air. The pressure level is together with then cautiously restored past times admitting air therefore that the score of the liquid inward the 2 limbs of the U-tube becomes equal.
Under these weather the pressure level read on the barometric leg when subtracted from the atmospheric pressure level gives the vapor pressure level of the liquid at the temperature of the experiment because the pressure level of the vapor inward the bulb Influenza A virus subtype H5N1 is balanced past times the pressure level of air introduced to convey the liquid inward the 2 limbs of the U-tube at the same level.