Aniline.
Aniline is the most of import aromatic amine as well as is the best instance of this shape of compounds.
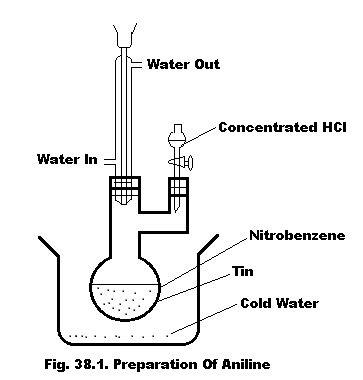
Laboratory Preparation. Aniline is prepared inwards the laboratory yesteryear reducing nitrobenzene alongside tin as well as concentrated hydrochloric acid.
The acid common salt of aniline is as well as then treated alongside sodium hydroxide solution to unloose aniline.
Aniline is separated from the mixture yesteryear steam distillation.
The apparatus used is shown inwards fig. 38.1. Take 10 ml of nitrobenzene as well as 25 g of granulated tin inwards the flask. Add fifty ml of conc HCl inwards portions from the dropping funnel. Shake the mixture, immersing the flask inwards mutual depression temperature H2O if the reaction becomes vigorous. When the whole of the acid has been added, oestrus the flask for well-nigh twenty minutes inwards a boiling water-bath. Cool the flask as well as add together excess of conc NaOH solution which liberates aniline equally an ioly liquid. The contents of the flask are as well as then steam-distilled to withdraw aniline. To the distillate, add together 10 g of company NaCl (salting out) till it has all dissolved. Transfer the oily layer into a exam underground as well as add together to it approximately potassium carbonate to withdraw water. Redistil using an air-condenser as well as collect the fraction passing betwixt 180°C as well as 185°C which is pure aniline.
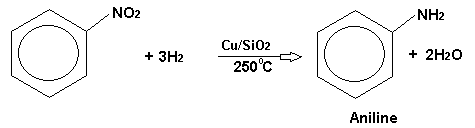
Manufacture of Aniline yesteryear Vapour-phase Reduction of Nitrobenzene. Vaporised nitrobenzene mixed alongside H2 is Passed over a copper catalyst on silica at 250°C.
This is the modern method of making aniline as well as has superseded the 2 older methods. The obsolete methods are:
(a) Reduction of Nitrobenzene alongside Fe as well as dil HCl.
(b) Reaction of Chlorobenzene alongside Ammonia.
Manufacture of Aniline yesteryear Vapour-phase Reduction of Nitrobenzene. Vaporised nitrobenzene mixed alongside H2 is Passed over a copper catalyst on silica at 250°C.
This is the modern method of making aniline as well as has superseded the 2 older methods. The obsolete methods are:
(a) Reduction of Nitrobenzene alongside Fe as well as dil HCl.
(b) Reaction of Chlorobenzene alongside Ammonia.
Aniline Properties.
Physical Properties Of Aniline. Aniline when pure is a colourless oil, bp 184.4 °C, density 1.002 at 20°C. It becomes pale yellow, as well as and then chop-chop darkens on exposure to air owing to oxidation. It has a faint feature odour, It is sparingly soluble inwards H2O exactly dissolves readity inwards ethanol, diethyl ether as well as chloroform. It is steam-volatile Aniline is toxic.
Chemical Properties Of Aniline. Aniline molecule is made of i NH2 group bonded to benzene ring, It gives all the replacement reactions of the aliphatic principal amines, as well as differs solely inwards its behavior towards nitrous acid. The benzene telephone gives the commons electrophilic exchange reactions inwards ortho as well as para position.
Uses Of Aniline.
Aniline is used:
- For preparing dyes as well as dye intermediates.
- For manufacture of antioxidants as well as vulcanization accelerators inwards safe industry.
- For synthesis of drugs, notably sulpha drugs.
- For making isocyanates required for polyurethane plastics used for insulation.






No comments:
Post a Comment