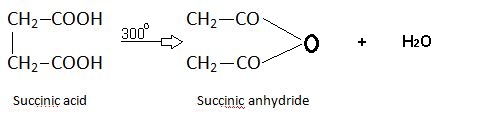
Succinic Acid.
Succinic acid was offset obtained equally a effect of distillation of amber together with thus its elevate (Latin,
Succinum = amber). It is besides produced during the alcoholic fermentation of sugar.
Succinic acid preparation.
(1) Succinic acid is prepared from ethylene bromide yesteryear reacting alongside sodium cyanide together with subsequent hydrolysis of ethylene cyanide.
Succinic acid tin flame besides live on synthesized alongside the assistance of Malonic Ester Synthesis.
Succinic acid properties.
Physical properties. Succinic acid forms white monoclinic prisms, mp 188°C. It is soluble inward ethyl alcohol together with ether, only moderately soluble inward water,
Chemical properties. Succinic acid gives all the normal reactions of a dicarboxylic acid.
(2) Reaction alongside Ammonia. With ammonia it forms ammonium succinate wich upon heating loses a molecule of ammonia to yield succinimide.
When succinimide is treated alongside an alkaline metal solution of Br2 at 0°C, it forms N-bromosuccinimide (NBS) which is a valuable reagent for allylic bromination.
(3) Electrolysis of Potassium Salt. The electrolysis of a rigid solution of potassium succinate gives ethylene.
Succinic acid uses.
Succinic acid is used.
- In the industry of lacquers together with dyes.
- in volumetric analysis equally a criterion heart for acid-base titrations.
Succinic acid structure.
Chemical Names of Succinic acid. Butanedioic acid. Succinic acid. 1,4-Butanedioic acid.Succinic acid is
dicarboxylic acid.The Molecular Formula of Succinic acid is : C4H6O4. The tooth of Succinic acid.118.088 g.mol-1.
Succinic acid hazards.
Succinic acid tin flame drive irritation of the skin, mucous, eyes membranes together with upper respiratory tract.





No comments:
Post a Comment