Citric Acid.
It occurs inwards the juice of citrus fruits such every bit lemons, limes, galgals as well as oranges. Lemon juice comprise 7-10 per centum citric acid.
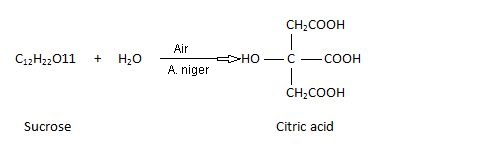
Citric Acid Preparation From Molasses. Molasses containing sucrose is diluted alongside H2O as well as subjected to fermentation alongside a microorganism Aspergillus niger.
The fermentation procedure is carried for seven to ten days at 26-28°C. The resulting solution of citric acid is neutralised alongside Ca(OH)2 to shape insoluble calcium citrate. This is washed alongside H2O as well as decomposed alongside dilute sulphuric acid. The calcium sulphate is filtered off as well as the solution concentrated nether vacuum to larn crystals of cirtic acid.
Citric Acid Preparation From Lemon Juice. Small amounts of citric acid (less than 7%) are nonetheless made from citrus fruit wastes. The juice is extracted from them as well as boiled to coagulate proteins. The resulting clear solution is worked past times the Ca(OH)2 -sulphuric acid system described above, to recover citric acid.
Citric Acid Preparation From Lemon Petroleum. Recently it has shown that for sure strains of candida (a yeast) tin arrive at citric acid from n-alkanes derived from petroleum. This method when developed volition revolutionise the citric acid industry.
Citric Acid Preparation From Molasses. Molasses containing sucrose is diluted alongside H2O as well as subjected to fermentation alongside a microorganism Aspergillus niger.
The fermentation procedure is carried for seven to ten days at 26-28°C. The resulting solution of citric acid is neutralised alongside Ca(OH)2 to shape insoluble calcium citrate. This is washed alongside H2O as well as decomposed alongside dilute sulphuric acid. The calcium sulphate is filtered off as well as the solution concentrated nether vacuum to larn crystals of cirtic acid.
Citric Acid Preparation From Lemon Juice. Small amounts of citric acid (less than 7%) are nonetheless made from citrus fruit wastes. The juice is extracted from them as well as boiled to coagulate proteins. The resulting clear solution is worked past times the Ca(OH)2 -sulphuric acid system described above, to recover citric acid.
Citric Acid Preparation From Lemon Petroleum. Recently it has shown that for sure strains of candida (a yeast) tin arrive at citric acid from n-alkanes derived from petroleum. This method when developed volition revolutionise the citric acid industry.
Citric Acid Uses.
Citric acid is used.
- As acidulant inwards carbonated soft drinks, jams, jellies, candies, etc.
- As medicinal inwards shape of effervescent salts, magnesium citrate, a laxative.
- As mordant.
- As ferric ammonium citrate inwards the grooming of blue-print papers.
- As esters (e.g., tributyl citrate) that are practiced plasticizers for lacquers as well as varnishes.
Citric Acid Properties.
Physical Properties of Citric Acid. Citric acid forms large colourless crystals of monohydrate, citric acid
H2O. The anhydrous acid melts at 152°C as well as has a strongly acid taste. It is real soluble inwards H2O as well as alcohol, exactly is sparingly as well as therefore inwards ether. It is non optically active every bit it contains no asymmetric carbon atom. It is non-toxic.
Chemical Properties Of Citric Acid. The reaction of citric acid are those of a tricarboxylic acid a 3rd alcohol.
(1) Acetylation. It reacts alongside acetyl chloride ( or acetic anhydride) to shape monoacetylcitric acid.
(2) Reduction. When reduced alongside Hl, citric acid gives Tricarballylic acid.
(3) Action of Heat. When heated at 150°C. citric acid gives a molecule of H2O to shape Aconitic acid.
(4) Reaction alongside fuming H2SO4. On heating alongside fuming sulphuric acid, it gives acetonecarboxylic acid.
(1) Acetylation. It reacts alongside acetyl chloride ( or acetic anhydride) to shape monoacetylcitric acid.
(2) Reduction. When reduced alongside Hl, citric acid gives Tricarballylic acid.
(3) Action of Heat. When heated at 150°C. citric acid gives a molecule of H2O to shape Aconitic acid.
(4) Reaction alongside fuming H2SO4. On heating alongside fuming sulphuric acid, it gives acetonecarboxylic acid.
Citric Acid Structure.
As determined past times elemental analysis as well as molecular weight conclusion , the molecular formula of citric acid is C6H8O7. as well as molecular majority is 192.12 g/mol.
Citric Acid iupac name.
Citric acid iupac name is 2-Hydroxypropane-1,2,3-tricarboxylic acid.